Latest publications
Nature Neuroscience (2025)
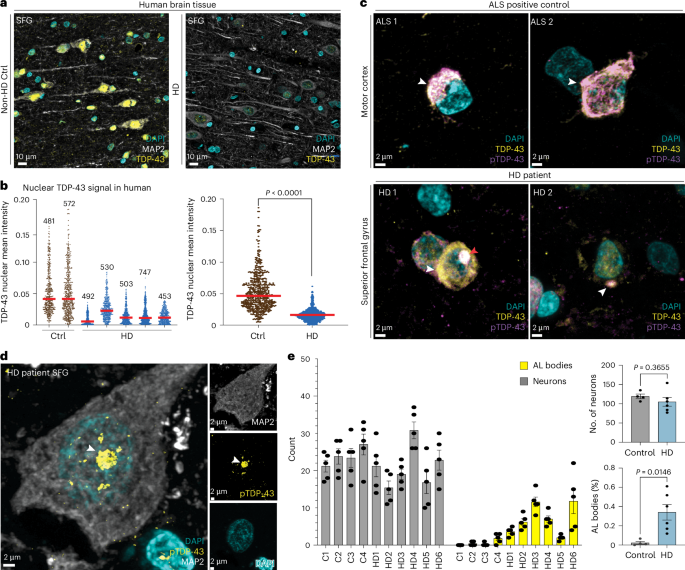
Huntington’s disease (HD) is caused by a CAG repeat expansion in the HTT gene, leading to altered gene expression. However, the mechanisms leading to disrupted RNA processing in HD remain unclear. Here we identify TDP-43 and the N6-methyladenosine (m6A) writer protein METTL3 to be upstream regulators of exon skipping in multiple HD systems. Disrupted nuclear localization of TDP-43 and cytoplasmic accumulation of phosphorylated TDP-43 occurs in HD
Neuronal STING activation in amyotrophic lateral sclerosis and frontotemporal dementia
Acta Neuropathologica (2024)
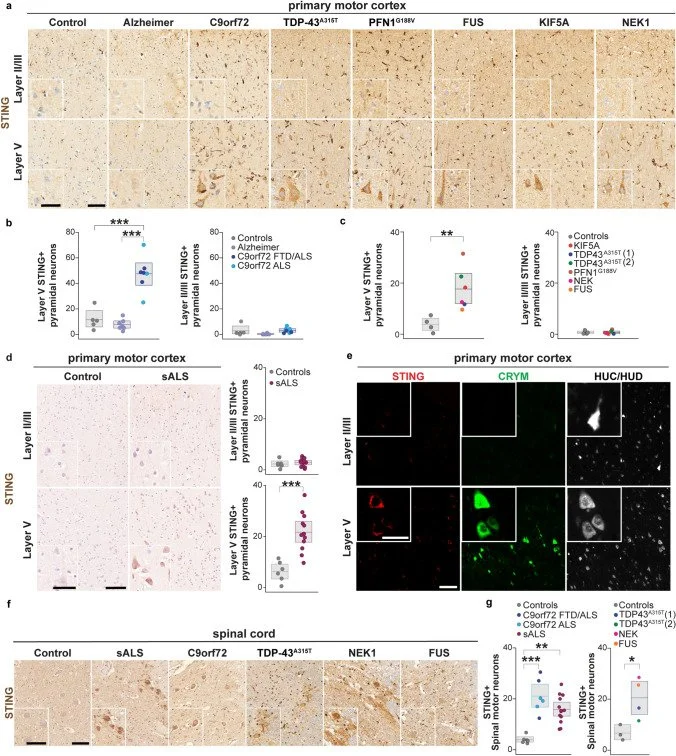
The stimulator of interferon genes (STING) pathway has been implicated in neurodegenerative diseases, including Parkinson’s disease and amyotrophic lateral sclerosis (ALS). While prior studies have focused on STING within immune cells, little is known about STING within neurons. Here, we document neuronal activation of the STING pathway in human postmortem cortical and spinal motor neurons from individuals affected by familial or sporadic ALS….
All publications
Recurrent patterns of widespread neuronal genomic damage shared by major neurodegenerative disorders
Zinan Zhou, Lovelace J. Luquette, Guanlan Dong, Junho Kim, Jayoung Ku, Kisong Kim, Mingyun Bae, Diane D. Shao, Bezawit Sahile, Michael B. Miller, August Yue Huang, William J. Nathan, Andre Nussenzweig, Peter J. Park, Clotilde Lagier-Tourenne, Eunjung Alice Lee, Christopher A. Walsh
BioRxiv (2025) DOI: https://www.biorxiv.org/content/10.1101/2025.03.03.641186v3
Thai B. Nguyen, Ricardo Miramontes, Carlos Chillon-Marinas, Roy Maimon, Sonia Vazquez-Sanchez, Alice L. Lau, Nicolette R. McClure, Zhuoxing Wu, Keona Q. Wang, Whitney E. England, Monika Singha, Jennifer T. Stocksdale, Marie Heath, Ki-Hong Jang, Sunhee Jung,Karen Ling, Paymann Jafar-nejad, Jharrayne I. McKnight, Leanne N. Ho, Osama Al Dalahmah, Richard L. M. Faull, Joan S. Steffan, Jack C. Reidling, Cholsoon Jang, Gina Lee, Don W. Cleveland, Clotilde Lagier-Tourenne, Robert C. Spitale & Leslie M. Thompson
Nature Neuroscience (2025) DOI: https://doi.org/10.1038/s41593-024-01850-w
Gina Picchiarelli* , Anne Wienand*, Salim Megat, Amr Aly, Marije Been, Nibha Mishra, Saskia Hutten, Erin Sternburg, Pierre Cauchy, Stéphane Dieterle , Marica Catinozzi, Valérie Demais, Laura Tzeplaeff, Annemarie Huebers , Dagmar Zeuschner, Angela Rosenbohm, Albert C. Ludolph, Anne-Laurence Boutillier, Tobias Boeckers, Dorothee Dormann, Maria Demestre, Chantal Sellier, Clotilde Lagier-Tourenne, Erik Storkebaum, Luc Dupuis
BioRxiv (2024) DOI: : https://doi.org/10.1101/2024.09.18.613669
Neuronal STING activation in amyotrophic lateral sclerosis and frontotemporal dementia
Christine Marques, Aaron Held, Katherine Dorfman, Joon Sung, Catherine Song, Amey S. Kavuturu, Corey Aguilar, Tommaso Russo, Derek H. Oakley, Mark W. Albers, Bradley T. Hyman, Leonard Petrucelli, Clotilde Lagier‐Tourenne, Brian J. Wainger
Acta Neuropathologica (2024) DOI: https://doi.org/10.1007/s00401-024-02688-z
Ana Rita Agra Almeida Quadros*, Zhaozhi Li, Xue Wang, I Sandra Ndayambaje, Sandeep Aryal, Nandini Ramesh, Matthew Nolan, Rojashree Jayakumar, Yi Han, Hannah Stillman, Corey Aguilar, Hayden J Wheeler, Theresa Connors, Jone Lopez-Erauskin, Michael W Baughn, Ze'ev Melamed, Melinda S Beccari, Laura Olmedo Martínez, Michael Canori, Chao-Zong Lee, Laura Moran, Isabelle Draper, Alan S Kopin, Derek H Oakley, Dennis W Dickson, Don W Cleveland, Bradley T Hyman, Sudeshna Das, Nilüfer Ertekin-Taner*, Clotilde Lagier-Tourenne*
Acta Neuropathologica (2024) DOI: https://doi.org/10.1007/s00401-023-02655-0
Zinan Zhou, Junho Kim, August Yue Huang, Matthew Nolan, Junseok Park, Ryan Doan, Taehwan Shin, Michael B. Miller, Brian Chhouk, Katherine Morillo, Rebecca C. Yeh, Connor Kenny, Jennifer E. Neil, Chao-Zong Lee, Takuya Ohkubo, John Ravits, Olaf Ansorge, Lyle W. Ostrow, Clotilde Lagier-Tourenne*, Eunjung Alice Lee*, Christopher A. Walsh*
BioRxiv (2023) DOI: https://doi.org/10.1101/2023.11.30.569436
Thai B Nguyen, Ricardo Miramontes, Carlos Chillon-Marinas, Roy Maimon, Sonia Vazquez-Sanchez, Alice L Lau, Nicolette R McClure, Whitney E England, Monika Singha, Jennifer T Stocksdale, Ki-Hong Jang, Sunhee Jung, Jharrayne I McKnight, Leanne N Ho, Richard L M Faull, Joan S Steffan, Jack C Reidling, Cholsoon Jang, Gina Lee, Don W Cleveland, Clotilde Lagier-Tourenne, Robert C Spitale, Leslie M Thompson
BioRxiv (2023) DOI: 10.1101/2023.10.31.565004
Aaron Held, Michelle Adler, Christine Marques, Charles Jourdan Reyes, Amey S Kavuturu, Ana R A A Quadros, I Sandra Ndayambaje, Erika Lara, Michael Ward, Clotilde Lagier-Tourenne, Brian J Wainger
Cell Report (2023) DOI: 10.1016/j.celrep.2023.113046
Jone López-Erauskin, Mariana Bravo-Hernandez, Maximiliano Presa, Michael W Baughn, Ze'ev Melamed, Melinda S Beccari, Ana Rita Agra de Almeida Quadros, Olatz Arnold-Garcia, Aamir Zuberi, Karen Ling, Oleksandr Platoshyn, Elkin Niño-Jara, I Sandra Ndayambaje, Melissa McAlonis-Downes, Larissa Cabrera, Jonathan W Artates, Jennifer Ryan, Anita Hermann, John Ravits, C Frank Bennett, Paymaan Jafar-Nejad, Frank Rigo, Martin Marsala, Cathleen M Lutz*, Don W Cleveland*, Clotilde Lagier-Tourenne*
Nature Neuroscience (2023) DOI: 10.1038/s41593-023-01496-0
Mechanism of STMN2 cryptic splice-polyadenylation and its correction for TDP-43 proteinopathies
Michael W Baughn*, Ze'ev Melamed*, Jone López-Erauskin, Melinda S Beccari, Karen Ling, Aamir Zuberi, Maximilliano Presa, Elena Gonzalo-Gil, Roy Maimon, Sonia Vazquez-Sanchez, Som Chaturvedi, Mariana Bravo-Hernández, Vanessa Taupin, Stephen Moore, Jonathan W Artates, Eitan Acks, I Sandra Ndayambaje, Ana R Agra de Almeida Quadros, Paayman Jafar-Nejad, Frank Rigo, C Frank Bennett, Cathleen Lutz, Clotilde Lagier-Tourenne*, Don W Cleveland*
Science (2023) DOI: 10.1126/science.abq5622
Meenakshi Sundaram Kumar, Karly M. Stallworth, Anastasia C. Murthy, Su Min Lim, Nan Li, Aastha Jain, James B Munro, Nicolas L. Fawzi, Clotilde Lagier-Tourenne, Daryl A. Bosco
Journal of Molecular Biology (2023) https://doi.org/10.1016/j.jmb.2023.167972
Gasdermin-E mediates mitochondrial damage in axons and neurodegeneration
Dylan V Neel, Himanish Basu, Georgia Gunner, Matthew D Bergstresser, Richard M. Giadone, Haeji Chung, Rui Miao, Vicky Chou, Eliza M. Brody, Xin Jiang, Edward B. Lee, Christine Marques, Aaron Held, Brian Wainger, Clotilde Lagier-Tourenne, Yong-Jie Zhang, Leonard Petrucelli, Tracy L. Young-Pearse, Alice S Chen-Plotkin, Lee L. Rubin, Judy Lieberman, Isaac M Chiu
Neuron (2023) https://doi.org/10.1016/j.neuron.2023.02.019
Aaron Held, Michelle Adler, Christine Marques, Amey S. Kavuturu, Ana R.A.A. Quadros, I. Sandra Ndayambaje, Erika Lara, Michael Ward, Clotilde Lagier-Tourenne, Brian J. Wainger
bioRxiv (2022)10.25.513780; doi: https://doi.org/10.1101/2022.10.25.513780
Jone Lopez-Erauskin, Mariana Bravo-Hernandez, Maximiliano Presa, Michael W. Baughn, Ze’ev Melamed, Melinda S. Beccari, Ana Rita Agra de Almeida Quadros, Aamir Zuberi, Karen Ling, Oleksandr Platoshyn, Elkin Niño-Jara, I. Sandra Ndayambaje, Olatz Arnold-Garcia, Melissa McAlonis-Downes, Larissa Cabrera, Jonathan W. Artates, Jennifer Ryan, Frank Bennett, Paymaan Jafar-nejad, Frank Rigo, Martin Marsala, Cathleen M. Lutz*, Don W. Cleveland*, Clotilde Lagier-Tourenne*
bioRxiv (2022) 12.11.519794; doi: https://doi.org/10.1101/2022.12.11.519794
Melanie Jambeau, Kevin D. Meyer, Marian Hruska-Plochan, Ricardos Tabet, Chao-Zong Lee, Ananya Ray-Soni, Corey Aguilar, Kitty Savage, Nibha Mishra, Nicole Cavegn, Petra Borter, Chun-Chia Lin, Karen Jansen-West, Jay Jiang, Fernande Freyermuth, Nan Li, Pierre De Rossi, Manuela Pérez-Berlanga, Xin Jiang, Lilian M. Daughrity, Joao Pereira, Sarav Narayanan, Yuanzheng Gu, Shekhar Dhokai, Isin Dalkilic-Liddle, Zuzanna Maniecka, Julien Weber, Michael Workman, Melissa McAlonis-Downes, Eugene Berezovski, Yong-Jie Zhang, James Berry, Brian J. Wainger, Mark W. Kankel, Mia Rushe, Christoph Hock, Roger M. Nitsch, Don W. Cleveland, Leonard Petrucelli, Tania Gendron, Fabio Montrasio, Jan Grimm*, Magdalini Polymenidou*, Clotilde Lagier-Tourenne*
Proceedings of the National Academy of Sciences (2022) https://doi.org/10.1073/pnas.2123487119
Loss of TDP-43 in male germ cells causes meiotic failure and impairs fertility in mice
Campbell KM., Xu Y., Patel C., Rayl JM., Zomer HD., Osuru HP., Pratt M., Pramoonjago P., Timken., Miller LM., Ralph A., Storey KM., Peng Y., Drnevich J., Lagier-Tourenne C., Wong PC., Qiao H., Reddi PP.
J. Biol. Chem. (2021) Sep 29:101231.
Wild-type FUS corrects ALS-like disease induced by cytoplasmic mutant FUS through autoregulation
Sanjuan-Ruiz I, Govea-Perez N., McAlonis-Downes M., Dieterle S., Megat S., Dirrig-Grosch S., Picchiarelli G., Piol D., Zhu Q., Myers B., Lee CZ., Cleveland DW., Lagier-Tourenne C., Da Cruz S.*, Dupuis L.*
Molecular Neurodegeneration. (2021) 16(1):61.
Jelena Scekic-Zahirovic, Inmaculada Sanjuan-Ruiz, Vanessa Kan, Salim Megat, Pierre De Rossi, Stéphane Dieterlé, Raphaelle Cassel, Marguerite Jamet, Pascal Kessler, Diana Wiesner, Laura Tzeplaeff, Valérie Demais, Sonu Sahadevan, Katharina M. Hembach, Hans-Peter Muller, Gina Picchiarelli, Nibha Mishra, Stefano Antonucci, Sylvie Dirrig-Grosch, Jan Kassubek, Volker Rasche, Albert Ludolph, Anne-Laurence Boutillier, Francesco Roselli, Magdalini Polymenidou, Clotilde Lagier-Tourenne, Sabine Liebscher* & Luc Dupuis*.
Nature Communications. (2021) May 21;12(1):3028. doi: 10.1038/s41467-021-23187-9.
Zhu Q, Jiang J, Gendron TF, McAlonis-Downes M, Jiang L, Taylor A, Diaz Garcia S, Ghosh Dastidar S, Rodriguez MJ, King P, Zhang Y, La Spada AR, Xu H, Petrucelli L, Ravits J, Da Cruz S, Lagier-Tourenne C*, Cleveland DW*.
Nat Neurosci. (2020) May;23(5):615-624. doi: 10.1038/s41593-020-0619-5. Epub 2020 Apr 13. PMID: 32284607
Phenotypic Suppression of ALS/FTD-Associated Neurodegeneration Highlights Mechanisms of Dysfunction.
Bartoletti M, Bosco DA, Da Cruz S, Lagier-Tourenne C, Liachko N, Markmiller S, Webster KM, Wharton KA.J
Journal of Neurosci. (2019) Oct 16;39(42):8217-8224. doi: 10.1523/JNEUROSCI.1159-19.2019. PMID: 31619490
Picchiarelli G, Demestre M, Zuko A, Been M, Higelin J, Dieterlé S, Goy MA, Mallik M, Sellier C, Scekic-Zahirovic J, Zhang L, Rosenbohm A, Sijlmans C, Aly A, Mersmann S, Sanjuan-Ruiz I, Hübers A, Messaddeq N, Wagner M, van Bakel N, Boutillier AL, Ludolph A, Lagier-Tourenne C, Boeckers TM, Dupuis L, Storkebaum E.
Nat Neurosci. (2019) Nov;22(11):1793-1805. doi: 10.1038/s41593-019-0498-9. Epub 2019 Oct 7.PMID: 31591561
Disruption of RNA Metabolism in Neurological Diseases and Emerging Therapeutic Interventions.
Nussbacher JK, Tabet R, Yeo GW*, Lagier-Tourenne C*.
Neuron. (2019) Apr 17;102(2):294-320. doi: 10.1016/j.neuron.2019.03.014. PMID: 30998900
Overriding FUS autoregulation in mice triggers gain-of-toxic dysfunctions in RNA metabolism and autophagy-lysosome axis.
Ling SC*, Dastidar SG, Tokunaga S, Ho WY, Lim K, Ilieva H, Parone PA, Tyan SH, Tse TM, Chang JC, Platoshyn O, Bui NB, Bui A, Vetto A, Sun S, McAlonis-Downes M, Han JS, Swing D, Kapeli K, Yeo GW, Tessarollo L, Marsala M, Shaw CE, Tucker-Kellogg G, La Spada AR, Lagier-Tourenne C, Da Cruz S, Cleveland DW*.
Elife. (2019) Feb 12;8. pii: e40811. doi: 10.7554/eLife.40811.
Melamed Z, López-Erauskin J, Baughn MW, Zhang O, Drenner K, Sun Y, Freyermuth F, McMahon MA, Beccari MS, Artates JW, Ohkubo T, Rodriguez M, Lin N, Wu D, Bennett CF, Rigo F, Da Cruz S, Ravits J, Lagier-Tourenne C*, Cleveland DW*.
Nat Neurosci. (2019) Feb;22(2):180-190. doi: 10.1038/s41593-018-0293-z. Epub 2019 Jan 14.
López-Erauskin J, Tadokoro T, Baughn MW, Myers B, McAlonis-Downes M, Chillon-Marinas C, Asiaban JN, Artates J, Bui AT, Vetto AP, Lee SK, Le AV, Sun Y, Jambeau M, Boubaker J, Swing D, Qiu J, Hicks GG, Ouyang Z, Fu XD, Tessarollo L, Ling SC, Parone PA, Shaw CE, Marsala M, Lagier-Tourenne C, Cleveland DW*, Da Cruz S*.
Neuron. (2018) Nov 21;100(4):816-830.e7. doi: 10.1016/j.neuron.2018.09.044. Epub 2018 Oct 18.
Taking on the Elephant in the Tissue Culture Room: iPSC Modeling for Sporadic ALS.
Wainger BJ*, Lagier-Tourenne C*.
Cell Stem Cell. (2018) Oct 4;23(4):466-467. doi: 10.1016/j.stem.2018.09.015.
Animal models of neurodegenerative diseases.
Dawson TM*, Golde TE*, Lagier-Tourenne C*.
Nat Neurosci. (2018) Oct;21(10):1370-1379. doi: 10.1038/s41593-018-0236-8. Epub 2018 Sep 24. Review.
Guo L, Kim HJ, Wang H, Monaghan J, Freyermuth F, Sung JC, O'Donovan K, Fare CM, Diaz Z, Singh N, Zhang ZC, Coughlin M, Sweeny EA, DeSantis ME, Jackrel ME, Rodell CB, Burdick JA, King OD, Gitler AD, Lagier-Tourenne C, Pandey UB, Chook YM, Taylor JP*, Shorter J*.
Cell. (2018) Apr 19;173(3):677-692.e20. doi: 10.1016/j.cell.2018.03.002.
Nuclear pores: the gate to neurodegeneration.
Li N, Lagier-Tourenne C.
Nat Neurosci. (2018) Feb;21(2):156-158. doi: 10.1038/s41593-017-0066-0. No abstract available.
Ricardos Tabet, Laure Schaeffer, Fernande Freyermut, Melanie Jambeau, Michael Workman, Chao-Zong Lee, Chun-Chia Lin, Jie Jiang, Karen Jansen-West, Hussein Abou-Hamdan, Laurent Désaubry, Tania Gendron, Leonard Petrucelli, Franck Martin*, Clotilde Lagier-Tourenne*
Nat Commun. (2018) 11;9(1):152.
Novel autosomal dominant TNNT1 mutation causing nemaline myopathy.
Konersman CG, Freyermuth F, Winder TL, Lawlor MW, Lagier-Tourenne C, Patel SB.
Mol Genet Genomic Med. (2017) 5(6):678-691.
Gasset-Rosa F., Chillon-Marinas C., Goginashvili A., Atwal R.S., Artates J.W., Tabet R., Wheeler V.C., Bang A.G., Cleveland D.W.*. Lagier-Tourenne C.*
Neuron (2017) 94(1):48-57.
Scekic-Zahirovic J., Oussini H.E., Mersmann S., Drenner K., Wagner M., Sun Y., Allmeroth K., Dieterle S., Sinniger J., Dirrig-Grosch S., Rene R., Dormann D., Haass C., Ludolph A.C., Lagier-Tourenne C., Storkebaum E.*, Dupuis L*.
Acta Neuropathologica (2017) doi:10.1007/s00401-017-1687-9.
Jiang J., Zhu Q., Gendron TF., Saberi S., McAlonis-Downes M., Seelman A., Stauffer JE., Jafar-Nejad P., Drenner K., Schulte D., Chun S., Sun S., Ling SC., Myers B., Engelhardt J., Katz M., Baughn M., Platoshyn O., Marsala M., Watt A., Heyser CJ., Ard MC., De Muynck L., Daughrity LM., Swing DA., Tessarollo L., Jung CJ., Delpoux A., Utzschneider DT., Hedrick SM., de Jong PJ., Edbauer D., Van Damme P., Petrucelli L., Shaw CE., Bennett CF., Da Cruz S., Ravits J., Rigo F., Cleveland DW.*, Lagier-Tourenne C.*
Neuron (2016) 90(3):535-550.
C9ORF72 poly(GA) aggregates sequester and impair HR23 and nucleocytoplasmic transport proteins.
Zhang YJ., Gendron TF., Grima JC., Sasaguri H., Jansen-West K., Xu YF., Katzman RB., Gass J., Murray ME., Shinohara M., Lin WL., Garrett A., Stankowski JN., Daughrity L., Tong J., Perkerson EA., Yue M., Chew J., Castanedes-Casey M., Kurti A., Wang ZS., Liesinger AM., Baker JD., Jiang J., Lagier-Tourenne C., Edbauer D., Cleveland DW., Rademakers R., Boylan KB., Bu G., Link CD., Dickey CA., Rothstein JD., Dickson DW., Fryer JD., Petrucelli L.
Nature Neuroscience (2016) 19(5):668-77.
Scekic-Zahirovic J., Sendscheid O., El Oussini H., Jambeau M., Sun Y., Mersmann S., Wagner M., Dieterlé S., Sinniger J., Dirrig-Grosch S., Drenner K., Birling M.C., Qiu J., Zhou Y., Li H., Fu X.D., Rouaux C., Shelkovnikova T., Witting A., Ludolph A.C., Kiefer F., Storkebaum E.*, Lagier-Tourenne C.*, Dupuis L*.
EMBO J (2016) 35(10):1077-97.
Sun S., Sun Y., Ling SC., Ferraiuolo L., McAlonis-Downes M., Zou Y., Drenner K., Wang Y., Ditsworth D., Tokunaga S., Kopelevich A., Kaspar B.K., Lagier-Tourenne C., Cleveland D.W.
PNAS (2015) 112:E6993-7002.
RNA-binding proteins in neurodegeneration: Seq and you shall receive.
Nussbacher J., Batra R., Lagier-Tourenne C.*, Yeo G.W.*
Trends Neurosci (2015) 38(4):226-236.
van Blitterswijk M., Gendron T.F., Baker M.C., DeJesus-Hernandez M., Finch N.A., Brown P.H., Daughrity L.M., Murray M.E., Heckman M.G., Jiang J., Lagier-Tourenne C., Edbauer D., Cleveland D.W., Josephs K.A., Parisi J.E., Knopman D.S., Petersen R.C., Petrucelli L., Boeve B.F., Graff-Radford N.R., Boylan K.B., Dickson D.W., Rademakers R.
Acta Neuropathol (2015) PMID: 26437865.
Gendron T.F., van Blitterswijk M., Bieniek K.F., Daughrity L.M., Jiang J., Rush B.K., Pedraza O., Lucas J.A., Murray M.E., Desaro P., Robertson A., Overstreet K., Thomas C.S., Crook J.E., Castanedes-Casey M., Rousseau L., Josephs K.A., Parisi J.E., Knopman D.S., Petersen R.C., Boeve B.F., Graff-Radford N.R., Rademakers R., Lagier-Tourenne C., Edbauer D., Cleveland D.W., Dickson D.W., Petrucelli L., Boylan K.B.
Acta Neuropathol (2015) 130(4):559-73.
Exome sequencing in amyotrophic lateral sclerosis identified risk genes and pathways.
Cirulli E.T., Lasseigne B.N., Petrovski S., Sapp P.C., Dion P.A, Couthouis J., Lu Y.F., Wang Q., Krueger B.J., Ren Z., Keebler J., Han Y., Levy S.E., Boone B.E., Wimbish J.R., Waite L.L., Jones A.L., Carulli J.P., Day-Williams A.G., Staropoli J.F., Xin W.W., Chesi A., Raphael A.R., McKenna-Yasek D., Cady J., Vianney de Jong J.M., Kenna K.P., Smith B.N., Topp S., Miller J., Gkazi A., FALS Sequencing Consortium, Al-Chalabi A, Van den Berg L.H., Veldink J., Silani V., Ticazzi N., Shaw C.E., Baloh R.H., Appel S., Simpson E., Lagier-Tourenne C., Pulst S.M., Gibson S., Trojanowski J.Q., Elman L., McCluskey L., Grossman M., Shneider N.A., Chung W.K., Ravits J.M., Glass J.D., Sims K.B., Van Deerlin V.M., Maniatis T., Hayes S.D., Ordureau A., Swarup S., Landers J., Baas F., Allen A.S., Bedlack R.S., Harper J.W., Gitler A.D., Rouleau G.A., Brown R., Harms M.B., Cooper G.M., Harris T., Myers R.M., Goldstein D.B.
Science (2015) 27;347(6229):1436-41.
Sun S., Ling S.C., Qiu J., Albuquerque C.P., Zhou Y., Tokunaga S., Li H., Qiu H., Bui A., Yeo G., Huang E.J., Eggan K., Zhou H., Fu X.D., Lagier-Tourenne C., Cleveland D.W.
Nat Commun (2015) 6:6171.
Hu J., Liu J., Yu D., Aiba Y., Lee S., Pendergraff H., Boubaker J., Artates J.W., Lagier-Tourenne C., Lima W.F., Swayze E.E., Prakash T.P., Corey D.R.
Nucleic Acid Ther (2014) 14(3):199-209.
Mutant Huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors.
Crotti A., Benner C., Kerman B.E., Gosselin D., Lagier-Tourenne C., Zuccato C., Cattaneo E., Gage F.H., Cleveland D.W., Glass C.K.
Nat Neurosci (2014) 17, 513-521.
Meyer K., Ferraiuolo L., Miranda C.J., Likhite S., McElroy S., Renusch S., Ditsworth D., Lagier-Tourenne C., Smith R.A., Ravits J., Burghes A.H., Shaw P.J., Cleveland D.W., Kolb S.J., Kaspar B.K.
PNAS (2014) 111:829-32.
Lagier-Tourenne C.*, Baughn M.*, Rigo F., Sun S., Liu P., Li H-R., Jiang J., Watt A., Chun S., Katz M., Qiu J., Sun Y., Ling S-C., Zhu Q., Polymenidou M., Drenner K., Artates J.W., McAlonis M.M., Markmiller S., Hutt R.R., Pizzo D.P., Cady J., Harms M.B., Baloh R.H., VandenBerg S.R., Yeo G.W, Fu X.D., Bennett C.F., Cleveland D.W., Ravits J.
PNAS (2013) 110:E4530-9.
Arnold E.S., Ling S.C., Huelga S.C., Lagier-Tourenne C., Polymenidou M., Ditsworth D., Kordasiewicz H.B., McAlonis-Downes M., Platoshyn O., Parone P.A., Da Cruz S., Clutario K.M., Swing D., Tessarollo L., Marsala M., Shaw C.E., Yeo G.W., Cleveland D.W.
PNAS (2013) 110, E736-45.
Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs.
Lagier-Tourenne C.*, Polymenidou M.*, Hutt K.R.*, Vu A.Q, Clutario K.M., Baughn M., Huelga S.C., Ling S.C., Liang T.Y., Mazur C., Wancewicz E., Salim A., Watt A., Freier S., Hicks G.G, Donohue J.P., Shiue L., Bennett C.F., Ravits J., Cleveland D.W., Yeo G.W.
Nat Neurosci (2012) 15, 1488-1497.
Misregulated RNA processing in amyotrophic lateral sclerosis.
Polymenidou M.*, Lagier-Tourenne C.*, Hutt K.R.*, Bennett C.F., Cleveland D.W, Yeo G.W.
Brain Res (2012) 1462, 3-15.
Polymenidou M.*, Lagier-Tourenne C.*, Hutt K.R.*, Huelga S.C., Moran J., Liang T.Y., Ling S.C., Sun E., Wancewicz E., Mazur C., Kordasiewicz H., Sedaghat Y., Donohue J.P., Shiue L., Bennett C.F., Yeo G.W., Cleveland D.W.
Nat Neurosci (2011) 14, 459-468.
Molecular diagnosis of known recessive ataxias by homozygosity mapping with SNP arrays.
H’mida-Ben Brahim D., M’zahem A., Assoum M., Bouhlal Y., Fattori F., Anheim M., Ali-Pacha L., Ferrat F., Chaouch M., Lagier-Tourenne C., Drouot N., Thibaut C., Benhassine T., Sifi Y., Stoppa-Lyonnet D., N’guyen K., Pouget J., Hamri A., Hentati F., Amouri R., Santorelli FM., Tazir M., Koenig M.
J Neurol (2011) 258, 56-67.
Assoum M., Salih M.A., Drouot N., H’Mida-Ben Brahim D., Lagier-Tourenne C., AlDrees A., Elmalik S.A., Ahmed T.S., Seidahmed M.Z., Kabiraj M.M., Koenig M.
Brain (2010) 133, 2439-47.
ALS-associated mutations in TDP-43 increase its stability and promote TDP-43 complexes with FUS/TLS.
Ling SC, Albuquerque CP, Han JS, Lagier-Tourenne C., Tokunaga S, Zhou H, Cleveland DW.
PNAS (2010) 107,13318-23.
Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency.
Quinzii CM, Lopez LC, Gilkerson RW, Dorado B, Coku J, Naini AB, Lagier-Tourenne C., Schuelke M, Salviati L, Carrozzo R, Santorelli F, Rahman S, Tazir M, Koenig M, Dimauro S, Hirano M.
FASEB J (2010) 24, 3733-43.
Braida C., Stefanatos RK., Adam B., Mahajan N., Smeets HJ., Niel F., Koenig M., Lagier-Tourenne C., Mandel JL., Faber CG., de Die-Smulders CE., Spaans F., Monckton DG.
Hum Mol Genet (2010) 19, 1399-412.
TDP-43 and FUS/TLS: emerging roles in RNA processing and neurodegeneration.
Lagier-Tourenne C.*, Polymenidou M.*, Cleveland DW.
Hum Mol Genet (2010) 19, R46-64.
Rethinking ALS: the FUS about TDP-43.
Lagier-Tourenne C., Cleveland D.W.
Cell (2009) 136, 1001-4.
Expanding CEP290 mutational spectrum in ciliopathies.
Travaglini L., Brancati F., Attie-Bitach T., Audollent S., Bertini E., Kaplan J., Perrault I., Iannicelli M., Mancuso B., Rigoli L., Rozet J.M., Swistun D., Tolentino J., Dallapiccola B., Gleeson J.G., Valente E.M., International JSRD Study Group, Zankl A., Leventer R., Grattan-Smith P., Janecke A., D’Hooghe M., Sznajer Y., Van Coster R., Demerleir L., Dias K., Moco C., Moreira A., Kim C.A., Maegawa G., Petkovic D., Abdel-Salam G.M., Abdel-Aleem A., Zaki M.S., Marti I., Quijano-Roy S., Sigaudy S., de Lonlay P., Romano S., Touraine R., Koenig M., Lagier-Tourenne C., et al.
Am J Med Genet (2009) 149A, 2173-2180.
Cossée M.*, Lagier-Tourenne C.*, Seguela C., Mohr M., Leturcq F., Gundesli H., Chelly J., Tranchant C., Koenig M., Mandel J.L.
Neuromusc Disord (2009) 19, 255-260.
SPG11 spastic paraplegia. Anew cause of juvenile parkinsonism.
Anheim M., Lagier-Tourenne C., Stevanin G., Fleury M., Durr A., Namer IJ., Denora P., Brice A., Mandel J.L., Koenig M., Tranchant C.
J Neurol (2009) 256, 104-108.
Lagier-Tourenne C., Tazir M., Lopez L.C., Quinzii C.M., Assoum M., Drouot N., Busso C., Makri S., Ali-Pacha L., Benhassine T., Anheim M., Lynch D.R., Thibault C., Plewniak F., Bianchetti L., Tranchant C., Poch O., DiMauro S., Mandel J.L., Barros M.H., Hirano M., Koenig M.
Am J Hum Genet (2008) 82, 661-672.
Homozygous mutation in SPATA16 is associated with male infertility in human globozoospermia.
Dam A.H., Koscinski I., Kremer J.A., Moutou C., Jaeger A.S., Oudakker A.R., Tournaye H., Charlet N., Lagier-Tourenne C., van Bokhoven H., Viville S.
Am J Hum Genet (2007) 81, 813-820.
Gribaa M., Salih M., Anheim M., Lagier-Tourenne C., H'Mida D., Drouot N., Mohamed A., Elmalik S., Kabiraj M., Al-Rayess M., Almubarak M., Betard C., Goebel H., Koenig M.
Brain (2007) 130, 1921-1928.
AHI1 gene mutations cause specific forms of Joubert syndrome-related disorders.
Valente E.M., Brancati F., Silhavy J.L., Castori M., Marsh S.E., Barrano G., Bertini E., Boltshauser E., Zaki M.S., Abdel-Aleem A., Abdel-Salam G.M., Bellacchio E., Battini R., Cruse R.P., Dobyns W.B., Krishnamoorthy K.S., Lagier-Tourenne C., Magee A., Pascual-Castroviejo I., Salpietro C.D., Sarco D., Dallapiccola B., Gleeson J.G., International JSRD Study Group.
Ann Neurol (2006) 59, 527-534.
Curbo S., Lagier-Tourenne C., Carrozzo R., Palenzuela L., Lucioli S., Hirano M., Santorelli F., Arenas J., Karlsson A., and Johansson M.
Genomics (2006) 87, 410-416.
The gene disrupted in Marinesco-Sjogren syndrome encodes SIL1, an HSPA5 cochaperone.
Anttonen A.K., Mahjneh I., Hamalainen R.H., Lagier-Tourenne C., Kopra O., Waris L., Anttonen M., Joensuu T., Kalimo H., Paetau A., Tranebjaerg L., Chaigne D., Koenig M., Eeg-Olofsson O., Udd B., Somer M., Somer H., Lehesjoki A.E.
Nat Genet (2005) 37, 1309-1311.
Thymidine phosphorylase mutations cause instability of mitochondrial DNA.
Hirano M., Lagier-Tourenne C., Valentino M.L., Marti R., and Nishigaki Y.
Gene (2005) 354, 152-6.
Homozygosity mapping of a third Joubert syndrome locus to 6q23.
Lagier-Tourenne C., Boltshauser E., Breivik N., Gribaa M., Betard C., Barbot C., and Koenig M.
J Med Genet (2004) 41, 273-277.
Lagier-Tourenne C., Ginglinger E., Alembik Y., De Saint Martin A., Peter M.O., Dulucq P., Jonveaux P., and Jeandidier E.
Am J Med Genet (2004) 125A, 77-85.
Homozygosity mapping of Marinesco-Sjogren syndrome to 5q31.
Lagier-Tourenne C., Tranebaerg L., Chaigne D., Gribaa M., Dollfus H., Silvestri G., Betard C., Warter J.M., and Koenig M.
Eur J Hum Genet (2003) 11, 770-778.
Biancalana V., Caron O., Gallati S., Baas F., Kress W., Novelli G., D’Apice M.R., Lagier-Tourenne C., Buj-Bello, A., Romero, N.B., Mandel, J.L.
Hum Genet (2003) 112, 135-142.
Lagier-Tourenne C., Chaigne D., Gong J., Flori J., Mohr M., Ruh D., Christmann D., Flament J., Mandel J.L., Koenig M., et al.
J Med Genet (2002) 39, 838-843.